Between 2017 and 2020, the obesity rate among American adults was 41.9%, while nearly 20% of American children and adolescents were classified as obese, according to the Centers for Disease Control and Prevention (CDC).
This alarming statistic highlights the growing need for effective weight loss solutions to combat the health risks associated with excess weight including diabetes, heart disease, and certain cancers. In response, pharmaceutical interventions like Zepbound and Wegovy have emerged as options for those struggling to lose weight through diet and exercise alone.
Together we’ll compare Zepbound and Wegovy, two medications approved for weight management, focusing on their efficacy, safety profiles, cost, and suitability for different patient populations.
[signup]
What is Wegovy?
Wegovy is one brand name of a generic drug called semaglutide. Semaglutide’s drug classes include antidiabetic, antiobesity agent, and endocrine-metabolic agent, and glucagon-like peptide-1 (GLP-1) receptor agonist.
What is the Difference Between Wegovy, Ozempic and Rybelsus?
Wegovy, Ozempic and Rybelsus are all brand names of the generic drug semaglutide.
While Wegovy is an injectable medication approved for weight management in obese or overweight adults, Ozempic is also an injectable but it is only approved for improving glycemic control in adults with type 2 diabetes and reducing cardiovascular risks.
Rybelsus, the first oral GLP-1 receptor agonist, is used for managing type 2 diabetes as an adjunct to diet and exercise.
How Wegovy Works
Wegovy works by activating the glucagon-like peptide-1 (GLP-1) receptors in the body, which are found mainly in the gastrointestinal tract, pancreas, and brain.
This activation enhances insulin release in response to high blood sugar after meals, slows down stomach emptying, and reduces the release of glucagon (a hormone that raises blood glucose levels).
It also interacts with brain receptors involved in hunger, reducing appetite and food cravings, which helps with weight loss. Semaglutide's long-acting nature is due to modifications that help it bind to proteins in the blood, allowing it to remain active in the body for an extended period.
Indications and Approved Uses
In 2021 the FDA approved Wegovy for chronic weight management in the following scenarios:
- In adults with a body mass index (BMI) of 30 or greater (obesity)
- or in adults with a BMI of 27 or greater (overweight) with at least one weight-related comorbidity such as hypertension, high cholesterol or type 2 diabetes
Wegovy Dosing and Administration
The dosing, frequency, and route of administration for Wegovy (semaglutide) are as follows:
- Route: Wegovy is administered as an injection under the skin.
- Frequency: Wegovy is given once weekly.
- Dosing Schedule: the dosing gradually increases over time to help reduce side effects. The schedule is as follows:
- 0.25 mg weekly for the first 4 weeks
- 0.5 mg weekly for weeks 5 to 8
- 1 mg weekly for weeks 9 to 12
- 1.7 mg weekly for weeks 13 to 16
- 2.4 mg weekly thereafter (maintenance dose)
This stepwise increase in dose allows patients to adjust to the medication, potentially reducing gastrointestinal side effects.
Wegovy Side Effects
Wegovy’s side effects include:
- Gastrointestinal (most common): nausea, vomiting, diarrhea, and decreased appetite are common; constipation and acid reflux may also occur.
- Kidney: risk of acute kidney injury, primarily from dehydration related to gastrointestinal issues.
- Skin: reactions at the injection site; infrequent severe allergic reactions have been reported.
- Pancreatitis: risk of pancreatitis (inflammation of the pancreas); blood tests may indicate elevated enzyme levels, sometimes without symptoms.
- Gallbladder: risk of gallstones (cholelithiasis) or gallbladder inflammation (cholecystitis), potentially linked to rapid weight loss.
- Eyes: in individuals with diabetic retinopathy, symptoms may temporarily worsen with rapid improvements in blood sugar control.
- Blood Sugar: mild risk of hypoglycemia, particularly in those using insulin or certain blood-glucose-lowering medications.
Wegovy Black Box Warning
Wegovy carries a black box warning:
- Thyroid C-Cell Tumors: animal studies have shown that semaglutide, the active ingredient in Wegovy, can cause thyroid C-cell tumors, including medullary thyroid carcinoma (MTC); however, it is unknown if this risk applies to humans. Wegovy is contraindicated for patients with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2 (MEN 2).
Efficacy and Clinical Trials
One clinical study involving 1,961 adults with obesity or overweight found that once-weekly semaglutide (Wegovy) at a dose of 2.4 mg, combined with lifestyle changes, resulted in an average weight loss of 14.9% over 68 weeks, significantly surpassing the 2.4% weight loss seen in the placebo group.
In another 12-week trial, Wegovy (semaglutide) significantly reduced appetite and energy intake, leading to an average weight loss of 5.0 kg primarily from fat mass, while improving control over eating and decreasing cravings for high-fat foods, all with good tolerability.
The STEP 5 trial found that once-weekly semaglutide 2.4 mg, combined with diet and lifestyle intervention, resulted in an average weight loss of 15.2% over 104 weeks in adults with obesity or overweight and a weight-related comorbidity, compared to 2.6% in the placebo group.
What is Zepbound?
Zepbound is the brand name of the generic medication tirzepatide. Tirzepatide’s drug classes include antidiabetic, endocrine-metabolic agent, and dual GLP-1 (glucagon-like peptide-1) and GIP (glucose-dependent insulinotropic polypeptide) receptor agonist.
What is the Difference Between Zepbound and Mounjaro?
Mounjaro and Zepbound are both brand name versions of the generic drug tirzepatide; however, only Zepbound is FDA-approved for weight loss, while Mounjaro is FDA-approved to lower blood sugar levels in type 2 diabetics.
How Zepbound Works
Zepbound (tirzepatide) is an injectable medication used for weight loss and blood sugar control in individuals with type 2 diabetes. It combines the effects of two hormones, GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1), to regulate appetite and metabolism.
Zepbound works by increasing insulin secretion while decreasing the production of glucagon, a hormone that raises blood sugar levels. It also slows gastric emptying, which helps patients feel full longer, and may reduce overall appetite, contributing to weight loss.
Zepbound Indications and Approved Uses
In late 2023 the FDA approved Zepbound for chronic weight management in the following scenarios:
- In adults with a body mass index (BMI) of 30 or greater (obesity)
- or in adults with a BMI of 27 or greater (overweight) with at least one weight-related comorbidity such as hypertension, high cholesterol or type 2 diabetes.
Zepbound Dosing and Frequency
The dosing, frequency, and route of administration for tirzepatide (Zepbound) are as follows:
- Route: tirzepatide is administered as an injection under the skin.
- Frequency: tirzepatide is given once weekly.
- Dosing Amounts: the available dosing strengths are 2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, and 15 mg per 0.5 mL. The initial starting dose is 2.5 mg once weekly, which is primarily for tolerance rather than glycemic control.
- Dose Escalation: after 4 weeks, the dose is increased to 5 mg. If additional glycemic control is needed, the dose may be escalated by 2.5 mg every 4 weeks up to a maximum of 15 mg weekly.
If a dose is missed, it can be administered within 4 days (96 hours) if possible; otherwise, the patient should skip the missed dose and resume the regular once-weekly schedule.
Zepbound Side Effects
Side effects of Zepbound include:
- Gastrointestinal (most common): nausea, vomiting, diarrhea, and decreased appetite are common; constipation and acid reflux may also occur.
- Heart: increased heart rate (sinus tachycardia), which may be influenced by other medications.
- Kidney: risk of kidney injury, mainly from dehydration due to gastrointestinal issues.
- Skin: reactions at the injection site; infrequent allergic reactions.
- Pancreatitis: risk of pancreatitis (inflammation of the pancreas); blood tests may show elevated enzyme levels, sometimes without symptoms.
- Gallbladder: risk of gallstones or gallbladder inflammation, possibly due to rapid weight loss.
- Eyes: in people with diabetic retinopathy, symptoms may temporarily worsen with quick blood sugar improvements.
- Blood Sugar: mild risk of low blood sugar (hypoglycemia), particularly in those using insulin or certain diabetes medications.
Zepbound Black Box Warning
Zepbound carries a black box warning:
- Thyroid C-Cell Tumors: animal studies have shown a potential for developing medullary thyroid carcinoma (MTC) with tirzepatide, although it’s unknown if this risk applies to humans. Tirzepatide is contraindicated for patients with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type-2 (MEN-2).
Efficacy and Clinical Trials
Zepbound has demonstrated efficacy in reducing blood sugar and body weight in clinical trials.
One phase 2 randomized, double-blind trial involving 316 participants evaluating tirzepatide (called LY3298176 during the trial) found that it significantly reduced HbA1c levels and body weight compared to dulaglutide and placebo, with gastrointestinal side effects being the most common adverse events.
Post hoc analyses of this study found that tirzepatide also improved insulin function and blood sugar control, as well as improved cholesterol and triglyceride profiles in people with type 2 diabetes.
The SURPASS-2 trial, which included 1,879 participants with type 2 diabetes, showed that tirzepatide was effective in a dose-dependent manner for reducing glycated hemoglobin (HbA1c) and body weight over 40 weeks, outperforming semaglutide (branded as Ozempic, Wegovy, and Rybelsus) in patients who had poorly controlled blood sugar despite metformin treatment.
Zepbound vs Wegovy: Key Differences
While both drugs offer promising weight loss effects, there are some important differences between Zepbound and Wegovy.
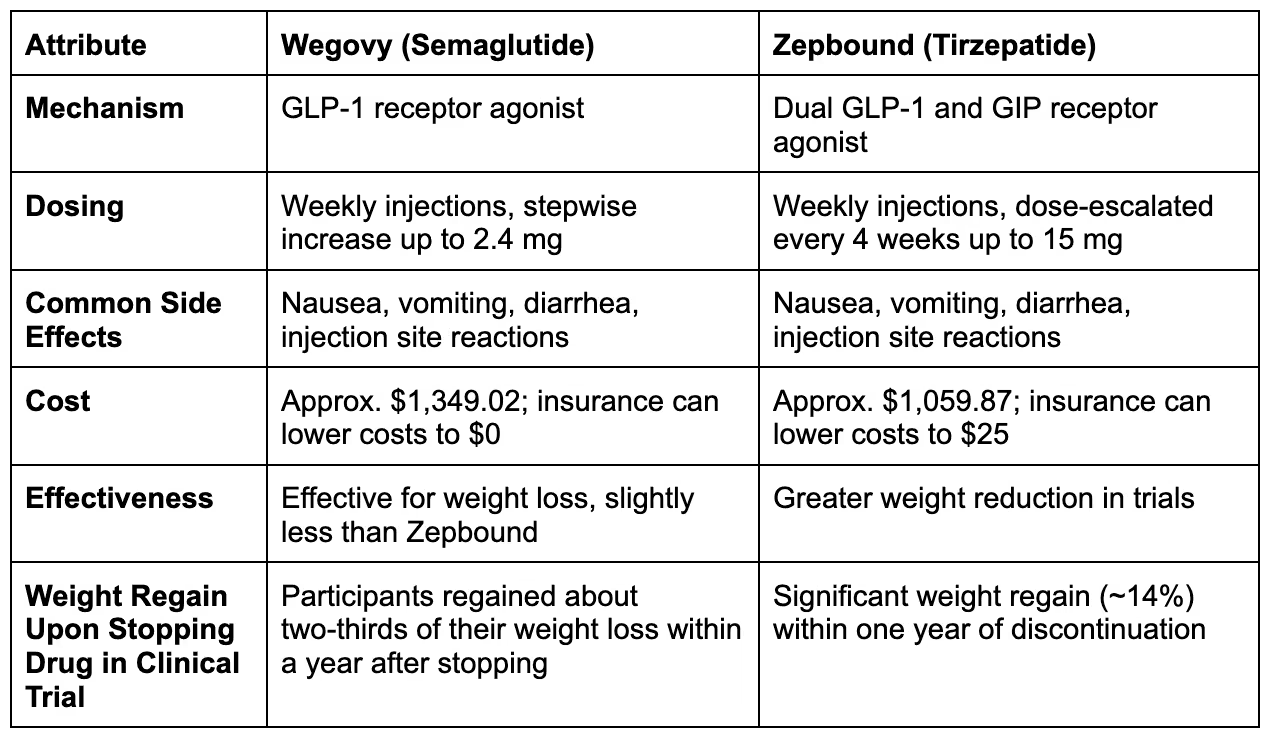
How Each Drug Works
Both Wegovy (semaglutide) and Zepbound (tirzepatide) are GLP-1 receptor agonists that enhance insulin secretion, reduce appetite, slow gastric emptying, and lower glucagon levels, leading to increased satiety and decreased food intake for weight loss.
Zepbound is also a GIP receptor agonist. As a GIP agonist, it enhances insulin sensitivity, boosts adiponectin levels, suppresses appetite more effectively, and improves pancreatic β-cell function, making it potentially more effective for managing obesity and type 2 diabetes than treatments targeting only GLP-1 receptors.
Dosage and Administration
Zepbound is administered as a subcutaneous injection, typically given once a week.
In contrast, Wegovy is also an injectable medication but comes with specific dosing schedules that start low and gradually increase based on patient tolerance. Both medications require regular administration, which may influence patient compliance.
Effectiveness: Which Is More Effective?
In clinical studies, both Zepbound and Wegovy have shown efficacy in promoting weight loss. However, Zepbound’s dual actions may provide greater benefit.
One study involving over 18,000 participants shows that tirzepatide leads to significantly greater weight loss than semaglutide, with patients on tirzepatide more likely to achieve clinically meaningful weight loss (≥5%, ≥10%, and ≥15%).
This effect was consistent in both patients with and without type 2 diabetes, and the rates of gastrointestinal side effects were similar for both medications.
In another 40-week phase 3 trial, tirzepatide (5 mg, 10 mg, and 15 mg) was found to be more effective than semaglutide (1 mg) in patients with type 2 diabetes not well managed by metformin. Participants on tirzepatide had larger reductions in HbA1c and greater weight loss than participants on semaglutide.
Cost and Insurance Coverage
Zepbound will be available in the U.S. by the end of 2023 in doses of 2.5 mg to 15 mg, with a list price of $1,059.87, approximately 20% lower than semaglutide's price.
Patients with insurance may pay as low as $25, while uninsured individuals could pay around $550 per month. Lilly plans to implement a savings card program to enhance access to Zepbound.
Wegovy® (semaglutide) injection has a list price of $1,349.02 per package. However, most patients do not pay this full amount due to insurance coverage for weight-management medications.
Insured patients may pay as little as $0 for a 28-day supply, while uninsured individuals might pay around $650.
The actual out-of-pocket cost for these medications depends on individual insurance plans and eligibility for discounts.
Which Results in More Weight Regain After Stopping The Drug?
Both Wegovy and Zepbound result in weight regain after stopping the drug.
An extension of the STEP 1 trial showed that after stopping semaglutide treatment, participants regained about two-thirds of their weight loss within a year.
The study included 1,961 adults with a BMI of 30 kg/m² or greater (or 27 kg/m² with a weight-related health issue) who underwent 68 weeks of treatment, followed by a year-long follow-up for 327 participants.
Similarly, people stopping Zepbound are likely to regain weight after stopping the drug.
The SURMOUNT-4 trial, involving 670 participants, found that adults with obesity or overweight regained much of their lost weight after stopping tirzepatide (Zepbound). Those who continued taking the drug maintained their weight loss and even lost more, while those who switched to a placebo regained about 14% of their body weight.
While these studies are large enough to demonstrate weight regain tendencies with these drugs, they also highlight the importance of ongoing treatment combined with lifestyle changes to help maintain weight loss.
[signup]
Key Takeaways
- The obesity rate among American adults reached 41.9% between 2017 and 2020, highlighting the urgent need for effective weight management solutions. Medications like Zepbound (tirzepatide) and Wegovy (semaglutide) offer new therapeutic options for individuals struggling with obesity and weight-related health issues.












%201.svg)










