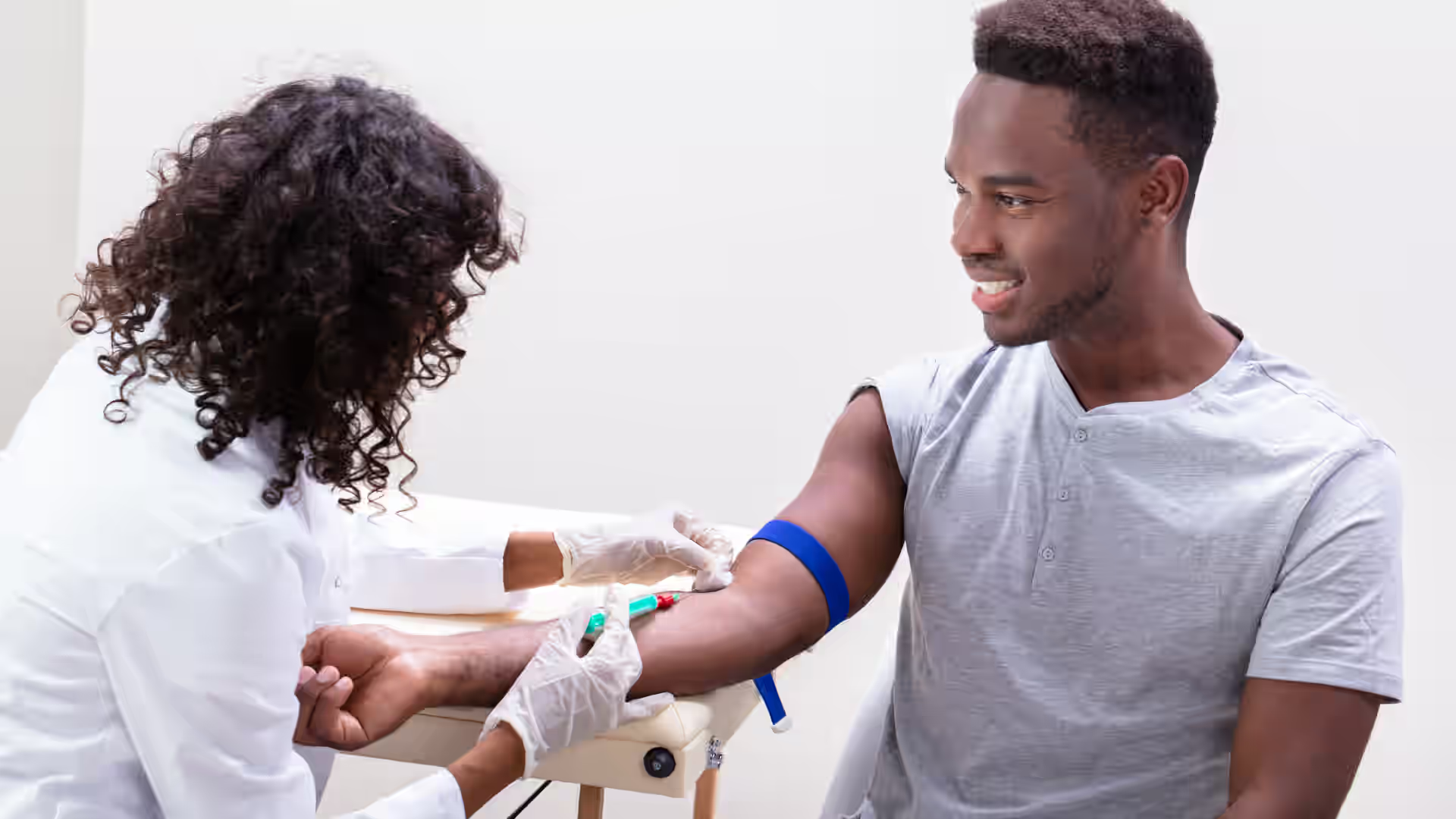
Diabetes insipidus (DI) is associated with a deficiency of antidiuretic hormone (ADH) or an insensitivity to its effects. This can lead to challenges in conserving water, which may result in excessive thirst (polydipsia) and the excretion of large volumes of dilute urine (polyuria).
Timely diagnosis and effective management of DI are important to help manage complications such as dehydration and electrolyte imbalances. This article aims to provide a comprehensive overview of DI, its symptoms, diagnosis, and management options to enhance awareness and support patient outcomes.
[signup]
What is Diabetes Insipidus?
When there is a deficiency in ADH or a reduced kidney response to ADH, the body may experience an imbalance in fluid regulation due to an inability to retain water effectively. This can result in excessive thirst (polydipsia) and the production of large volumes of dilute urine (polyuria) that characterizes diabetes insipidus. In DI, urine output is typically excessive, often exceeding 3 liters in 24 hours, with a very dilute concentration of osmolality less than 300 mOsm/kg.
Milestones in Understanding Diabetes Insipidus
The scientific understanding of DI has evolved significantly over several centuries since its first clinical description in the 18th century when patients were observed to have excessive thirst and urination without elevated blood glucose levels. In the early 20th century, researchers identified the hormone vasopressin (also known as ADH) and began to understand its important role in regulating water balance, bringing to light the intricacies of water balance in the body.
Differentiation from Diabetes Mellitus
While the names sound similar, diabetes insipidus and diabetes mellitus involve different hormonal imbalances and causes. Diabetes derives from the Greek word siphon, referring to urine, or the fluid filtered out by the kidneys. While both conditions cause frequent urination and excessive thirst, these symptoms are caused by distinct underlying mechanisms.
Excessive dilute urine occurs in DI due to ADH insufficiency or resistance that causes impaired water retention. On the other hand, in diabetes mellitus, impaired insulin production results in impaired glucose metabolism and high blood sugar levels that contribute to excessive thirst and urination.
Epidemiology of Diabetes Insipidus
DI is relatively rare, with an estimated prevalence of about 1 in 25,000 individuals. This condition occurs worldwide, with no specific geographic or ethnic predilection. There is no significant gender difference in the prevalence of DI, although some forms may show slight variations based on genetic factors.
DI can affect individuals of any age, but certain forms may have specific demographic patterns. DI arising from an issue with pituitary production of ADH (central DI) often arises in childhood or young adulthood, while DI occurring due to reduced kidney response to ADH (nephrogenic DI) can be congenital or acquired later in life.
Causes of Diabetes Insipidus
DI results from either a deficiency in ADH production (central DI) or a reduced kidney response to ADH (nephrogenic DI).
Central Diabetes Insipidus
Central DI, also referred to as arginine vasopressin (AVP)-deficiency, is the most common form of DI resulting from deficiency of the hormone AVP being released from the pituitary gland.
Idiopathic Central DI
Idiopathic central diabetes insipidus occurs when the deficiency in ADH production occurs with no identifiable underlying condition or obvious damage to the hypothalamus or pituitary gland.
Secondary Central DI
When identifiable damage to the hypothalamus or pituitary gland, such as from pituitary tumors, head trauma, autoimmune attack, infections, or surgeries, results in DI this is classified as secondary central diabetes insipidus. This damage to the posterior pituitary gland impairs ADH production, leading to symptoms of DI.
Nephrogenic Diabetes Insipidus
The less common nephrogenic DI occurs when resistance develops in the kidneys, causing them not to respond sufficiently when stimulated with ADH.
Inherited Nephrogenic DI
Inherited or hereditary nephrogenic DI is a genetic disorder where the kidneys are unable to respond to ADH. Normally, ADH binds to its type-2 receptor (AVPR2 )in the kidneys' collecting duct, causing the exocytosis of the water channel aquaporin 2 (AQP2) at the apical membrane, allowing water to be reabsorbed. Inherited nephrogenic DI results due to mutations in the AVPR2 gene or AQP2 gene, affecting the receptors or channels necessary for water reabsorption.
Acquired Nephrogenic DI
Acquired nephrogenic DI occurs when external factors such as certain medications (e.g., lithium), chronic kidney disease, or electrolyte imbalances (e.g., hypercalcemia, hypokalemia) impair the kidneys' response to ADH.
Dipsogenic Diabetes Insipidus
Primary Dipsogenic DI
Primary dipsogenic DI is a type of primary polydipsia involving an abnormal increase in thirst and fluid intake. This condition is due to a malfunction in the thirst mechanism in the hypothalamus, leading to excessive drinking and subsequent dilution of urine.
Secondary Dipsogenic DI
Secondary dipsogenic DI occurs due to brain injuries, surgeries, or diseases affecting the hypothalamus or other areas that regulate thirst. These injuries cause abnormal stimulation of thirst, leading to excessive fluid intake and diluted urine.
Gestational Diabetes Insipidus
Gestational DI occurs during pregnancy, often in the third trimester, due to increased metabolism of ADH by placental enzymes (vasopressinase) and sometimes due to increased ADH clearance. It is usually transient during pregnancy.
Symptoms of Diabetes Insipidus
Due to the inability of the kidneys to concentrate urine adequately, common symptoms of DI may include:
- excessive urination (polyuria)
- extreme thirst (polydipsia)
- frequent urination at night (nocturia)
In infants and children, symptoms of DI may manifest as irritability, poor feeding, failure to thrive, dehydration, dry skin, sunken fontanelles (soft spots on the head), lethargy, and delayed growth.
Pathophysiology
ADH, also known as vasopressin, is a hormone released from the posterior pituitary gland that normally helps the kidneys retain water by concentrating urine.
Dysfunction in the neurohypophyseal pathway involves impaired synthesis, release, or action of ADH in the hypothalamus or posterior pituitary gland due to damage to the hypothalamus or pituitary gland, leading to decreased production or release of ADH, or from defects in ADH receptors in the kidneys.
Renal tubular dysfunction occurs when the kidneys cannot respond to ADH appropriately, leading to decreased water reabsorption in the renal tubules. This can result from congenital defects in ADH receptors or acquired conditions such as electrolyte imbalances or medication-induced nephrogenic diabetes insipidus.
Risk Factors
Genetic Predispositions
Inherited or familial cases of DI can result from mutations in the AVPR2 or AQP2 genes that prevent the kidneys from responding to signals from ADH. Genetic counseling is crucial for individuals with a family history of DI to assess the risk of passing on genetic mutations and to understand potential inheritance patterns.
Environmental Factors
Certain environmental conditions, such as hot weather or high altitude, can exacerbate symptoms of DI by increasing fluid loss through sweating and evaporation. Individuals with DI should take precautions to stay hydrated and avoid overheating.
Medications
Exposure to medications such as lithium, demeclocycline, or antiretroviral drugs can contribute to the development of DI. Additionally, exposure to toxins or heavy metals may impair kidney function, leading to nephrogenic DI.
Medical Conditions
Medical conditions that affect the hypothalamus, pituitary gland, or kidneys, such as brain tumors, head trauma, or chronic kidney disease, are associated with an increased risk of developing DI.
Diagnosis and Tests
History and Physical
A thorough medical history and physical examination to identify underlying conditions, medications, or lifestyle factors that may contribute to DI are key steps in diagnosis.
Laboratory Testing
Laboratory testing to measure plasma and urine osmolality, or the concentration of solutes in blood and urine, helps to assess the kidney's ability to concentrate urine and overall hydration status.
In DI, urine osmolality is typically low despite dehydration while plasma osmolality is typically elevated due to dehydration. In addition, serum electrolytes, especially sodium, may become imbalanced due to excessive urination in DI. Elevated sodium (hypernatremia) is common due to excessive water loss through urination without adequate replacement although low sodium can also occur if fluid intake is excessively high in an attempt to compensate for water loss.
Water Deprivation Test
A water deprivation test involves restricting fluid intake while monitoring urine output and osmolality. There is an inability to concentrate urine in response to water deprivation in central or nephrogenic DI, so urine remains dilute (urine osmolality <300 mOsm/kg) even after prolonged dehydration, confirming the diagnosis. Giving desmopressin results in a rise in urine osmolality (>800 mOsm/kg) in central DI but not in nephrogenic DI.
Imaging Studies
Magnetic resonance imaging (MRI) or computed tomography (CT) imaging scans can identify structural abnormalities in the hypothalamus or pituitary gland, such as tumors or lesions, which may be associated with DI.
Complications
Potential acute complications of DI may include acute dehydration and electrolyte imbalances due to excessive urination, which can lead to symptoms such as weakness, dizziness, and confusion.
Over time, chronic complications may arise from untreated or poorly managed DI, including kidney damage, growth abnormalities in children, and cognitive impairments.
Overall, DI can significantly impact the quality of life and contribute to psychological distress, affecting daily activities and social interactions.
Medical Treatments and Lifestyle Management Techniques
Advances in genetic research, imaging techniques, and biochemistry in the latter part of the 20th century and into the 21st century have further refined the understanding of the mechanisms underlying this condition, leading to additional management options for DI.
Pharmacological Treatments
The medication desmopressin, a synthetic form of ADH, is commonly used for central DI, helping to reduce urine output and manage symptoms.
Medications such as thiazide diuretics or nonsteroidal anti-inflammatory drugs (NSAIDs) may be used in nephrogenic DI to help manage urine output by promoting sodium and water reabsorption in the kidneys.
Non-Pharmacological Treatments
Proper hydration strategies, such as carrying a water bottle and setting reminders to drink fluids, can help individuals with DI stay hydrated throughout the day. It is also important to monitor fluid intake and urine output to help prevent dehydration.
Dietary approaches, such as reducing salt (sodium) intake, can help manage electrolyte imbalances in individuals with DI.
Psychological support and counseling may be beneficial for individuals dealing with the emotional impact of living with DI. In addition, participating in peer support groups can provide valuable emotional support and practical advice for managing DI.
Pregnancy and Diabetes Insipidus Management
Management of gestational diabetes insipidus involves careful monitoring of fluid balance and the use of desmopressin, a synthetic ADH analog that is not degraded by placental enzymes. Close monitoring by healthcare providers helps ensure maternal and fetal health are maintained throughout pregnancy.
Outlook / Prognosis
The prognosis for individuals with DI varies depending on the underlying cause, severity of symptoms, and effectiveness of management. With appropriate management and support, most individuals with DI can lead normal lives and maintain a good quality of life.
Early diagnosis and management significantly improve outcomes, reducing the risk of complications such as dehydration and electrolyte imbalances. Other factors influencing prognosis include adherence to management plans, underlying health conditions, and access to healthcare resources for consistent monitoring and management.
[signup]
Key Takeaways
- Diabetes insipidus (DI) is a condition characterized by excessive thirst and urination due to a deficiency of ADH or the inability of the kidneys to respond to ADH, resulting in impaired water regulation.
- It can be classified into central DI (deficiency in antidiuretic hormone production), nephrogenic DI (kidney insensitivity to ADH), dipsogenic DI (abnormal thirst regulation), and gestational DI (occurs during pregnancy).
- Causes include damage to the hypothalamus or pituitary gland, genetic factors, certain medications, and environmental factors.
- Risk factors include a family history of DI, brain injuries, and exposure to medications like lithium.
- Symptoms of DI include excessive thirst, frequent urination, dehydration, electrolyte imbalances, and growth abnormalities in children.
- Complications may include kidney damage, cognitive impairment, and impact on quality of life.
- Diagnosis involves medical history, physical examination, laboratory tests (urine and plasma osmolality, serum electrolytes), water deprivation test, and imaging studies (MRI, CT scan).
- Pharmacological treatments include desmopressin to replace ADH in central DI and thiazide diuretics or NSAIDs to help manage symptoms of excessive urination and electrolyte imbalance in nephrogenic DI.
- Non-pharmacological treatments involve hydration strategies, dietary adjustments, and lifestyle modifications.
- With proper management, most individuals with DI can lead normal lives and maintain a good quality of life.
- Early diagnosis and management are crucial for improving outcomes and reducing the risk of complications.
- Future research may focus on identifying novel therapeutic targets, improving diagnostic tools, and exploring personalized management approaches to optimize outcomes for individuals with DI.











%201.svg)